
Note: The only difference between VALIUM10 and WALIUM10 is their selectable eye color marker VALIUM10 uses Vermilion, WALIUM10 uses White. Centrifuge (15,000 rpm, 20 min), wash once with 70% EtOH, and dissolve in 40ul ddH2O Primer used for DNA sequencing, Hsp70F: 5'-CGCAGCTGAACAAGCTAAAC-3'ġ2. Sequence hairpin construct to confirm the ftz intron is in the correct orientation (in our experience: 40-60% of the clones are correct). Select clone, culture in 5ml LB medium (Amp resistant) and miniprep At end elute with 100ul ddH2O.ġ0. Mix, incubate on ice 30 min, 42☌ heat shock, add SOC medium, incubate at 37☌ for 30 min, then plate (Amp resistant)ĩ. Mix, incubate at 25☌ for 1 hr, and then add 1ul proteinase K (From LR clonase kit), further incubate at 37☌ for 10 min to remove the recombinase. Recombination 45-85ng/ul pENTR/D-TOPO entry vector (from step 5) Oligo used for sequencing, M13-F: 5’-TTGTAAAACGACGGCCAGTC-3’ħ. Sequence pENTR/D-TOPO entry vector to ensure the insertion and its orientation is correct i.e., 3’ to 5’. Select clone, culture in LB medium (Kan resistant) and miniprep.Ħ. Transfer all ligation mix to 50ul TOP10 cells, place on ice for 30 min, heat shock, add SOC medium and incubate at 37☌ for 50 min, then plate (Kan resistant).ĥ. Mix, incubate at room temperature for 10 min PENTR/D-TOPO entry vector (Invitrogen, Cat.No. TOPO Ligation PCR product (there’s no need for purification) If multiple bands are seen, cut out the relevant band and purify it.ģ.
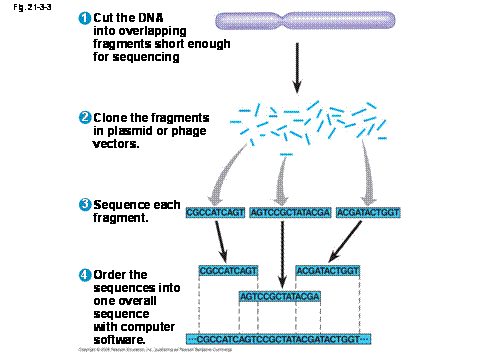
Once the PCR finishes (30 Cycles), load 10ul onto an agarose gel to verify the quality and quantity of the PCR product. Only splice-form universal portions of each gene were selected for oligo designĭNA Template (make sure to use genomic DNA or cDNA).19 bp predicted off-targets were avoided.The TRiP oligos were designed using SnapDragon with the following criteria:


 0 kommentar(er)
0 kommentar(er)
